Signaling systems consist of receptors that reside either in the cell membrane (surface receptors) or within the cells (in-tracellular receptors). Receptors are activated by a variety of extracellular signals or first messengers, including neuro-transmitters, protein hormones and growth factors, steroids, and other chemical messengers. Some lipid-soluble chemical messengers move through the membrane and bind to cytoplasmic or nuclear receptors to exert their physiologic effects. Signaling systems also include transducers and effectors that are involved in conversion of the signal into a physiologic response. The pathway may include additional intracellular mechanisms, called second messengers. Many molecules involved in signal transduction are proteins. A unique property of proteins that allows them to function in this way is their ability to change their shape or conformation, thereby changing their function and consequently the functions of the cell. Proteins often accomplish these
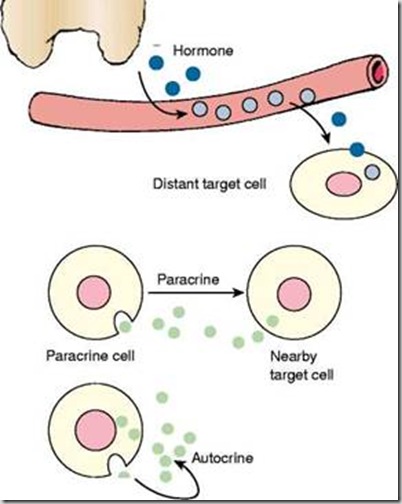
paracrine (B), and autocrine (C) secretions.
conformational changes through enzymes called protein kinases that catalyze the phosphorylation of amino acids in the protein structure.
Cell Surface Receptors
Each cell type in the body contains a distinctive set of surface receptors that enable it to respond to a complementary set of signaling molecules in a specific, preprogrammed
CELL COMMUNICATION
>- Cells communicate with each other and with the internal and external environments by a number of mechanisms, including electrical and chemical signaling systems that control electrical potentials, the overall function of a cell, and gene activity needed for cell division and cell replication.
>- Chemical messengers exert their effects by binding to cell membrane proteins or receptors that convert the chemical signal into signals within the cell, in a process called signal transduction.
> Cells can regulate their responses to chemical messengers by increasing or decreasing the number of active receptors on their surface.
way. These proteins are not static components of the cell membrane; they increase or decrease in number according to the needs of the cell. When excess chemical messengers are present, the number of active receptors decreases in a process called down-regulation; when there is a deficiency of the messenger, the number of active receptors increases through up-regulation. Three known classes of cell surface receptor proteins exist: G-protein linked, ion-channel linked, and enzyme linked.
G-Protein-Linked Receptors. With more than 1000 members, G-protein-linked receptors are the largest family of cell surface receptors. Although many intercellular messengers exist, they usually rely on the intermediary activity of a separate class of membrane-bound regulatory proteins to convert external signals (first messengers) into internal signals (second messengers). Because these regulatory proteins bind to guanine nucleotides such as guanine diphos-phate (GDP) and guanine triphosphate (GTP), they are called G proteins. G-protein-linked receptors mediate cellular responses for numerous types of first messengers, including proteins, small peptides, amino acids, and fatty acids derivatives such as the prostaglandins.
All G-protein-linked signal transduction systems rely on a series of orchestrated biochemical events (Fig. 4-11). They all have a receptor component, which functions as a signal discriminator by recognizing a specific first messenger, and they all undergo conformational changes with receptor binding that activate the G protein. The activated G protein, in turn, acts as a transducer, passing the message on to other membrane-bound intermediates called effectors. Often, the effector is an enzyme that converts an inactive precursor molecule into a second messenger, which diffuses into the cytoplasm and carries the signal beyond the cell membrane. A common second messenger is cyclic adenosine monophosphate (cAMP). It is activated by the enzyme adenyl cyclase, which generates cAMP by transferring phosphate groups from ATP to other proteins. This transfer changes the form and function of these proteins. Such changes eventually produce the cell response to the first messenger, whether it is a secretion, muscle contraction or relaxation, or a change in cell metabolism. Sometimes, it is the opening or closing of ion channels in the cell membrane.
Although there are differences among the G-protein-linked receptors, all share a number of features. All are found on the cytoplasmic side of the cell membrane, and all incorporate the GTPase cycle, which functions as the on-off switch for G-protein activity. GTPase is an enzyme that converts GTP with its three phosphate groups to GDP with its two phosphate groups. In the inactive state, G proteins contain tightly bound GDP. When a receptor coupled to a G protein is activated, the G protein releases the GDP and binds GTP. This causes the G protein to dissociate from the receptor and activate the effector. During the process of signal transduction, GTPase converts bound GTP to GDP, thereby returning the G protein to its resting state and switching the signal off. Certain bacterial toxins can bind to the G proteins, causing inhibition or stimulation of its signal function. One such toxin, the toxin of Vibrio
|
|
| Second Messenger |
cAMP cGMP
Inositol 1,4,5-trisphosphate and diacylglycerol
Cell Response |
IGURE4-11 Signal transduction pattern common to several second messenger systems. A protein or peptide hormone is the first messenger to a membrane receptor, stimulating or inhibiting a membrane-bound enzyme by means of a G protein. The amplifier enzyme catalyzes the production of a second messenger from a phosphorylated precursor. The second messenger then activates an internal effector, which leads to the cell response. (Redrawn from Rhoades R.A., Tanner G.A. [1996]. Medical physiology. Boston: Little, Brown)
cholerae, binds and activates the stimulatory G protein linked to the cAMP system that controls the secretion of fluid into the intestine. In response to the cholera toxin, these cells overproduce fluid, leading to severe diarrhea and life-threatening depletion of extracellular fluid volume.
Ion-Channel-LinkedReceptors. Ion-channel-linked receptors are involved in the rapid synaptic signaling between electrically excitable cells. A few neurotransmitters mediate this type of signaling by transiently opening or closing ion channels formed by integral proteins in the cell membrane. This type of signaling is involved in the transmission of impulses in nerve and muscle cells.
Enzyme-Linked Receptors. Many hormones, growth factors, and cytokines signal their target cells by binding to a class of receptors that activate an intracellular domain with enzyme (tyrosine kinase) activity. The enzyme catalyzes the phosphorylation of tyrosine residues in the receptor and target proteins, thereby transferring an external message to the cell interior. Enzyme-linked receptors mediate cellular responses such as calcium influx, increased sodium-potassium exchange, and stimulation of glucose
and amino acid uptake. Insulin acts by binding to a surface receptor with tyrosine kinase activity (see Chapter 43).
The signaling cascades generated by the activation of tyrosine kinase receptors are also involved in the function of growth factors. As their name implies, many growth factors are important messengers in signaling cell replacement and cell growth. Most of the growth factors belong to one of three groups: factors that foster the multiplication and development of various cell types (e.g., growth factor and epidermal growth factor); cytokines, which are important in the regulation of the immune system (see Chapter 19); and colony-stimulating factors, which regulate the proliferation and maturation of white and red blood cells (see Chapter 14).
![clip_image002[5] clip_image002[5]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhx_qGbQdhGbRQ2kHEx0EY6ZtgyjsYe72ypN51Bgw1TP7ZZDfvwvtvHqrpaHU3e6BDONC22l-fxNx9tjxIaNvZw4CdyTVtN0M9doHRHmBQOEpbaFaPOfnPa-oYB8FDaSUyQujWh4ot9oTM/?imgmax=800)

No comments:
Post a Comment